![]()
The Central Drugs Standard Control Organisation (CDSCO) has recently issued a significant alert, flagging over 50 medicines found to be substandard during routine tests conducted in August 2024. This revelation has raised concerns about the safety and efficacy of widely used medications, including those for common ailments such as acidity, high blood pressure, diabetes, and even essential vitamins and supplements. Here’s everything you need to know about the alert and its implications for public health.
Key Medications Flagged as Substandard
The list of medications flagged by CDSCO includes some of the most commonly prescribed drugs in India:
- Pan-D: Widely used to treat acidity and indigestion, Pan-D was among the drugs that failed the quality tests.
- Paracetamol: A popular pain reliever and fever reducer, this medication was also found to be substandard.
- Antibiotics: Essential for treating bacterial infections, antibiotics such as Amoxicillin and Potassium Clavulanate failed to meet the required quality standards.
- Blood Pressure Medications: Medications like Telmisartan, which is used to manage hypertension, were included in the list.
- Diabetes Medications: Glimepiride, commonly prescribed for Type-2 diabetes, was also flagged for substandard quality.
- Vitamins and Supplements: Drugs like Shelcal, a calcium and Vitamin D3 supplement, and Vitamin C and Vitamin B complex supplements were included as well.
List of Images of Drugs which are not of Standard Quality
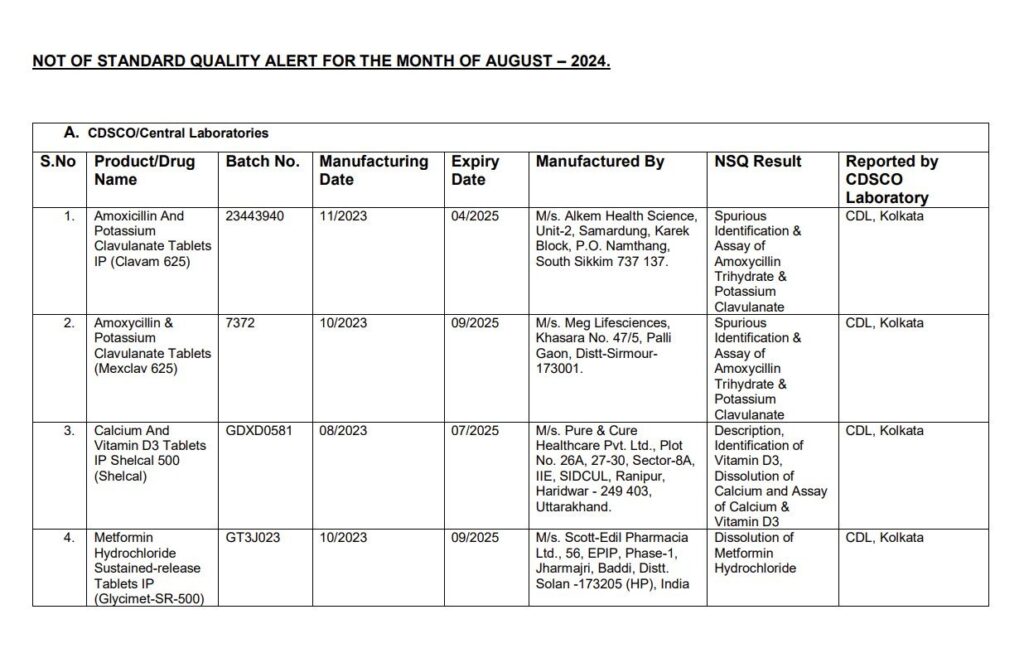
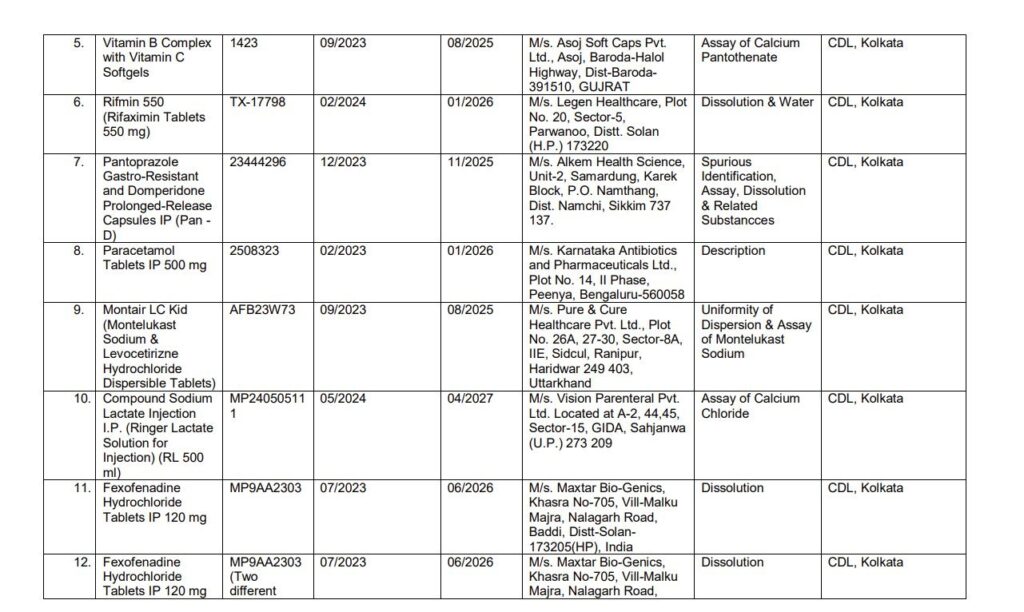
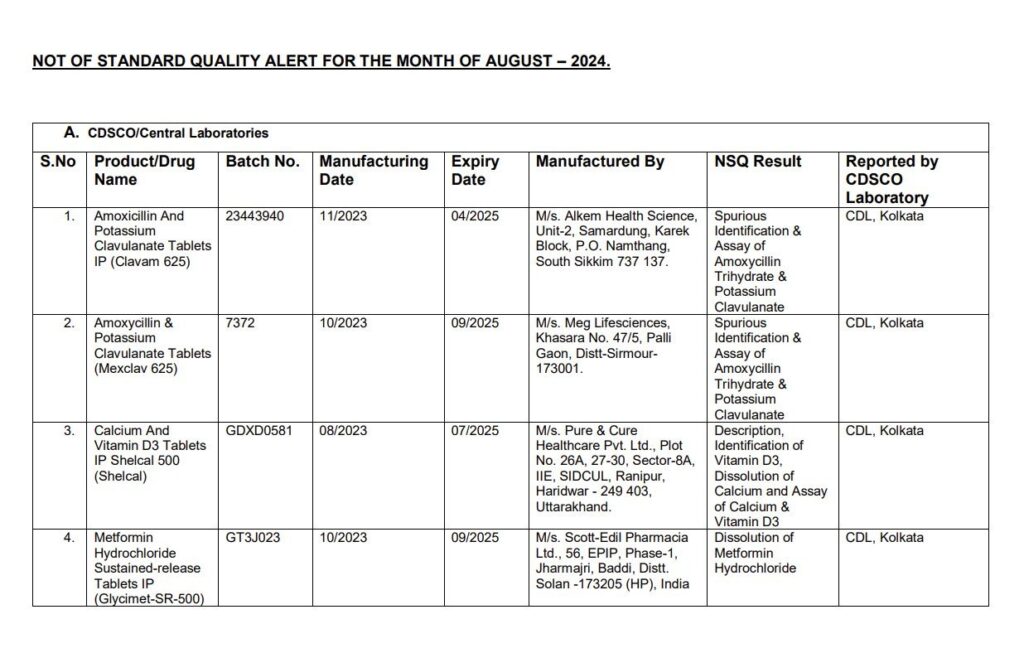
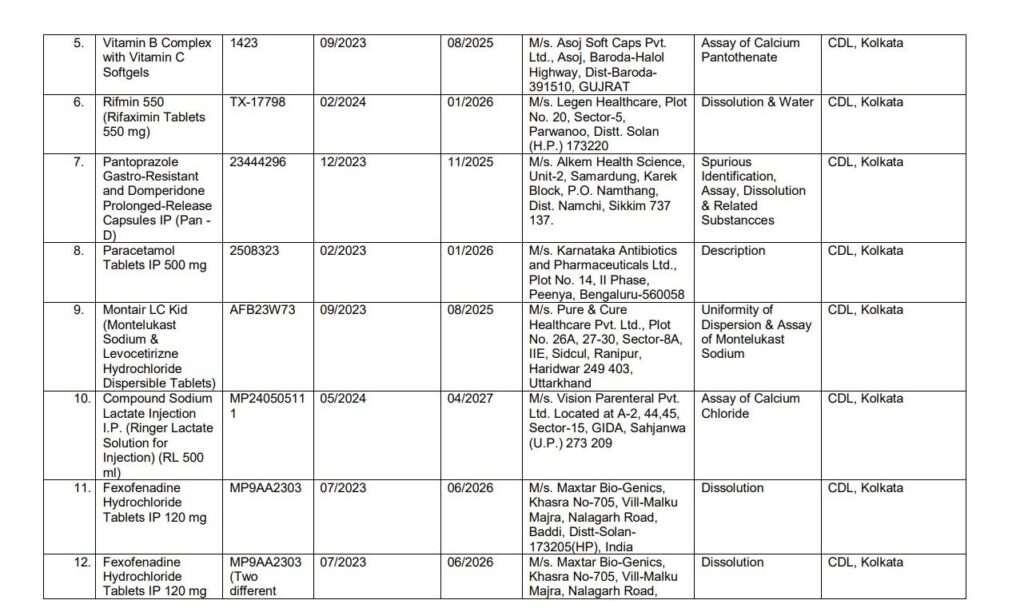
Manufacturers and Drugs Affected
The CDSCO’s report highlighted several reasons why these drugs were flagged as substandard:
- Failure in Dissolution Tests: Some drugs, such as those containing paracetamol, failed the dissolution test, meaning they did not break down properly in the body, which can compromise their effectiveness.
- Assay Failures: Several drugs have incorrect amounts of active ingredients, which can reduce their efficacy or lead to unintended side effects.
- Spurious Drugs: Certain medications were labeled as “spurious,” meaning they were counterfeit or manufactured under pretenses.
- Volume Uniformity: Issues in the uniformity of the drug’s volume were also cited in some cases, raising concerns about dosage accuracy.
What Happens Next?
Following this alert, the manufacturers of the flagged drugs are expected to recall the affected batches and investigate the cause of these quality failures. The CDSCO will continue its rigorous monitoring to ensure that the medications available in the Indian market meet the necessary safety and quality standards.
Additionally, healthcare providers and consumers are advised to stay informed about the medications they are using. It is always a good idea to check for updates from reliable sources or consult your doctor if you’re concerned about the safety of any medications you’re currently taking.
The Importance of Quality Control in Pharmaceuticals
This alert serves as a crucial reminder of the importance of stringent quality control in the pharmaceutical industry. Substandard medications not only jeopardize the health of individuals but also undermine public trust in healthcare systems. Regular testing, monitoring, and enforcement of quality standards are essential to ensuring that patients receive safe and effective treatments.
Final Thoughts
The recent CDSCO alert on substandard medicines has brought crucial attention to quality control challenges within the pharmaceutical industry. While the discovery of compromised medications may be concerning, it underscores the importance of regulatory oversight in maintaining drug safety standards. Consumers should remain informed and consult their healthcare providers if they have concerns regarding their prescribed medications.
For ongoing updates and detailed reports, referring to official CDSCO publications or consulting with medical professionals is highly recommended.
Related Article: List of Drugs Approved by CDSCO
For readers interested in learning about the approved medications by CDSCO, check out our comprehensive list that details the names, strengths, and indications of the drugs officially sanctioned by the regulatory body. This article provides insights into the various pharmaceutical products that meet the stringent quality and safety standards set by CDSCO, including those for chronic conditions and essential vitamins.
Read the full article here to stay informed about safe and approved medications in India.
